Which Describes How Elements Are Arranged in the Periodic Table
Vertical columns called groups where the elements have similar properties. He called the columns groups.
Periodic Table Science Quizizz
Which general area contains the nonmetallic.

. 1869 - two scientists determined a way to determine a way to put the elements in order it was lothar meyer and. In the periodic table the elements are arranged in columns and rows according to increasing atomic number see the table entitled Periodic Table. Modern Periodic Law- Modern Periodic Law states The chemical and physical.
What significance is there in the way the elements are arranged into vertical groups. The arrangement of elements in the Periodic table starts from the very first top left corner. Correct Answer - Option 2.
D Electrons are arranged in alphabetical order Medium Solution Verified by Toppr Correct option is A. The atomic number is the number of protons in an atom of that element. But hold on this was just a short answer.
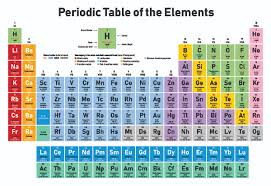
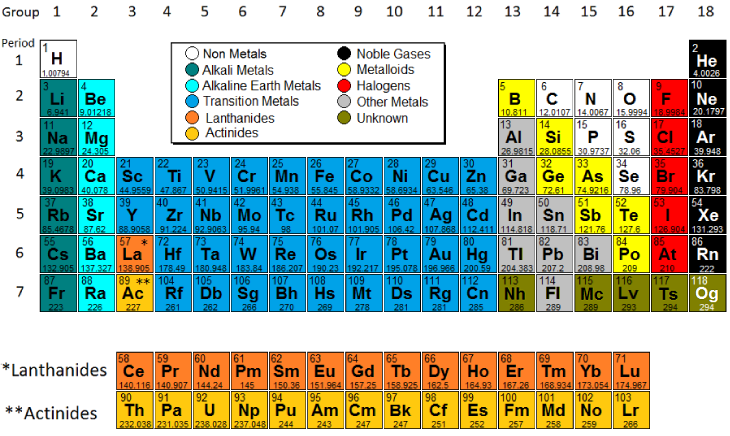
Yellow metals blue nonmetals and shaded green. Periodic Table - the ___ is an arrangemnt of chemical elements that is very helpful tool for determining thier chemical properties - the usefulness of the elements to us depneds on those properties - summarizes the relationship between elements - thE development of the ___ is the scientific method at work Valence Electrons. The rows are called periods.
Introductory Chemistry 6th Edition Edit edition Solutions for Chapter 5 Problem 9CRQ. Rows called periods in order of increasing atomic number. The columns of the table are called groups some of which have specific names such as the noble gases and the halogens.
What significance is there in the way the elements are arranged into vertical groups. Order generally coincides with increasing atomic mass. Rows called periods in order of increasing atomic number.
Which general area of the periodic table contains the metallic elements. Elements with the same number of electron shells are arranged in the horizontal rows periods and elements with similar properties are arranged in vertical columns groups. Elements having similar chemical properties and electronic structures appear in vertical columns groups.
How are the elements arranged in the table. Click here to know more about it. 211 describe the combustion of elements in oxygen including magnesium hydrogen and sulfur.
He assembled the table by classifying chemical elements in an order based on their periodicity of chemical and physical properties. Groups and Periods From left to right across each row elements are arranged by increasing atomic mass. Mendeleev discovered that if he placed eight elements in each row and then continued on to the next row the columns of the table would contain elements with similar properties.
Elements are placed in columns ie. Describe the periodic table of the elements. Atomic number and protons In the modern periodic table the elements are arranged according to their atomic number - not their relative atomic mass.
Now the periodic table has been arranged based on the atomic number of elements as it is believed that the atomic number is related to the properties of the elements. The elements in the Periodic Table are arranged in order of increasing atomic number. In the periodic table the elements are arranged into.
The periodic table got its name from the way the elements are arranged in rows which are called periods. Which general area of the periodic table contains the metallic elements. Atomic Number-Periodic Table- This is a table of chemical elements arranged in the order of atomic numbers in such a way that elements with the same atomic structure are displayed in the vertical column.
The organization of the periodic table allows you to predict the properties of the elements based on their position on the chart. In the periodic table the elements are arranged into. How can you locate a family of elements in the table.
C Elements are arranged by their valence electron configurations. How are the elements arranged in the modern periodic table. How are the elements arranged in the table.
The columns of the table are called groups some of which have specific names such as the noble gases and the halogens. As a bonus the Periodic Table also gives the average atomic mass of the each individual element. How are the elements of the periodic table.
B Elements are arranged by their atomic mass. Elements are arranged from left to right and top to bottom in order of increasing atomic number. There are 18 vertical columns or groups in the standard periodic table.
1864 - john newlands published his own version of the periodic table and developed the law of octaves. Elements in the periodic table are arranged in order of increasing atomic proton number. The arrangement starts from Doberneir law of triads then to Newlands law of octave then Mendleevs law where he arranged the elements based on their atomic weight.
Vertical columns called groups where the elements have similar properties. A Elements are arranged by their atomic numbers. Atomic Number The correct answer is Atomic Number.
How are elements arranged within the periodic table. 212 describe the formation of carbon dioxide from the thermal decomposition of metal carbonates including copperII carbonate. Which general area contains the nonmetallic elements.
The periodic law states that when elements are arranged in order of increasing atomic number there is a periodic repetition of physical and chemical properties. The s- p- and d-block elements of the periodic table are arranged into 18 numbered columns or groups. Describe the periodic table of the elements.
Periodic table ˌpɪərɪˈɒdɪk n Chemistry a table of the elements arranged in order of increasing atomic number based on the periodic law. In the modern periodic table the elements are arranged according to their atomic number not their relative atomic mass. Groups which reflects the number of valence electrons and then placed in rows in Periods which is a measure of the distance of the valence electrons ie the outermost electrons from the nuclear core.
How are elements grouped in the periodic table. Heres how it works. Elements in the same group have similar chemical properties because they have the.
The periodic table got its name from the way the elements are arranged in rows which are called periods. Rows called periods in order of increasing atomic number. At present there are three versions of the periodic table each with its own unique column headings in wide use.
The first element with atomic number 1 ie hydrogen is placed in the first cell then gradually the elements with atomic number 2 3 4 upto 118 are placed from the left to right in the Periodic table. Elements are listed in numerical order by atomic number. Solutions for Chapter 5 Problem 9CRQ.
So element number 1 hydrogen is the first element. Going down the periodic table the number of atomic orbitals increases by one for each row. Vertical columns called groups where the elements have similar properties.
In the modern periodic table the elements are arranged according to their atomic number not their relative atomic mass. The periodic table of elements arranges all of the known chemical elements in an informative array.
Periodic Table Model Science Software

What Is The Aufbau Principle Periodic Table Of The Elements Aufbau Principle Periodic Table

Comments
Post a Comment